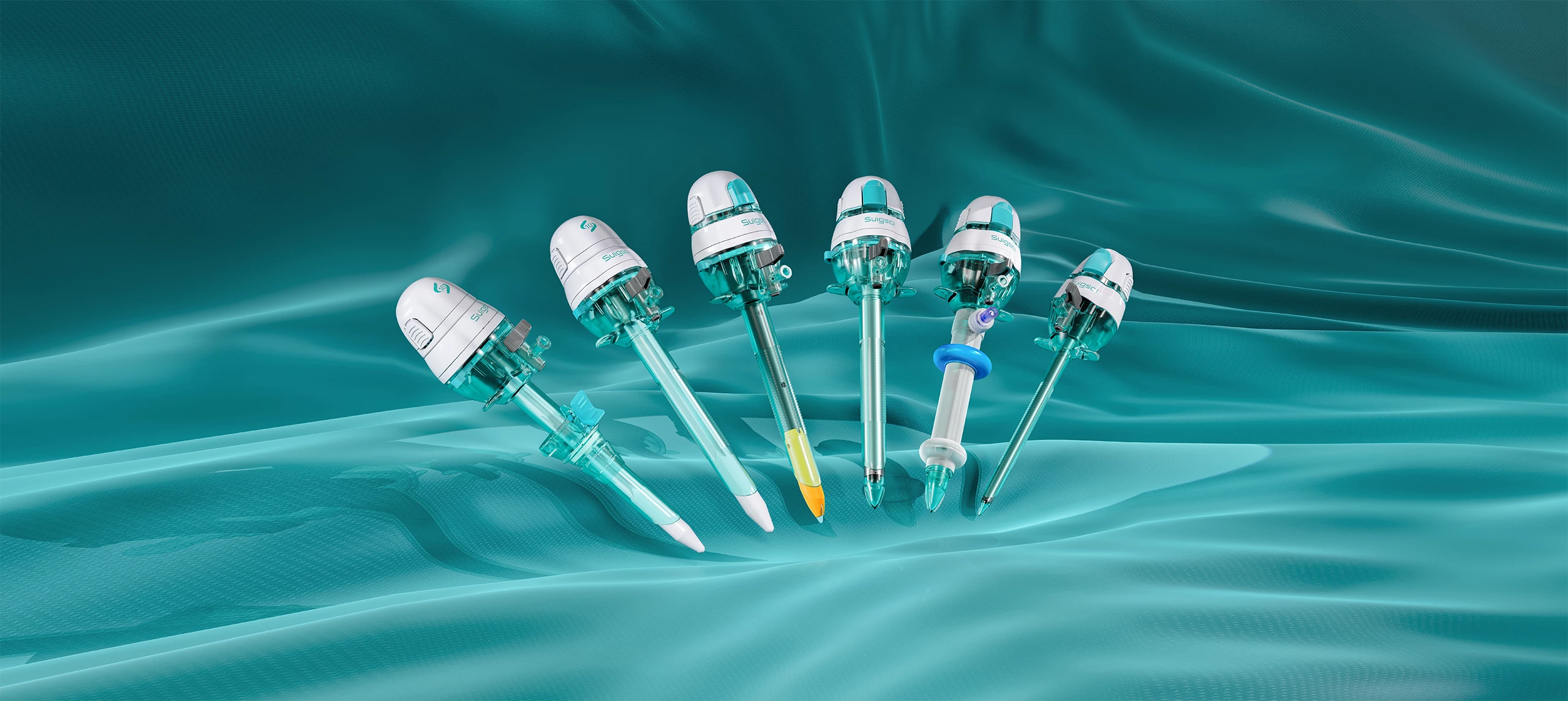
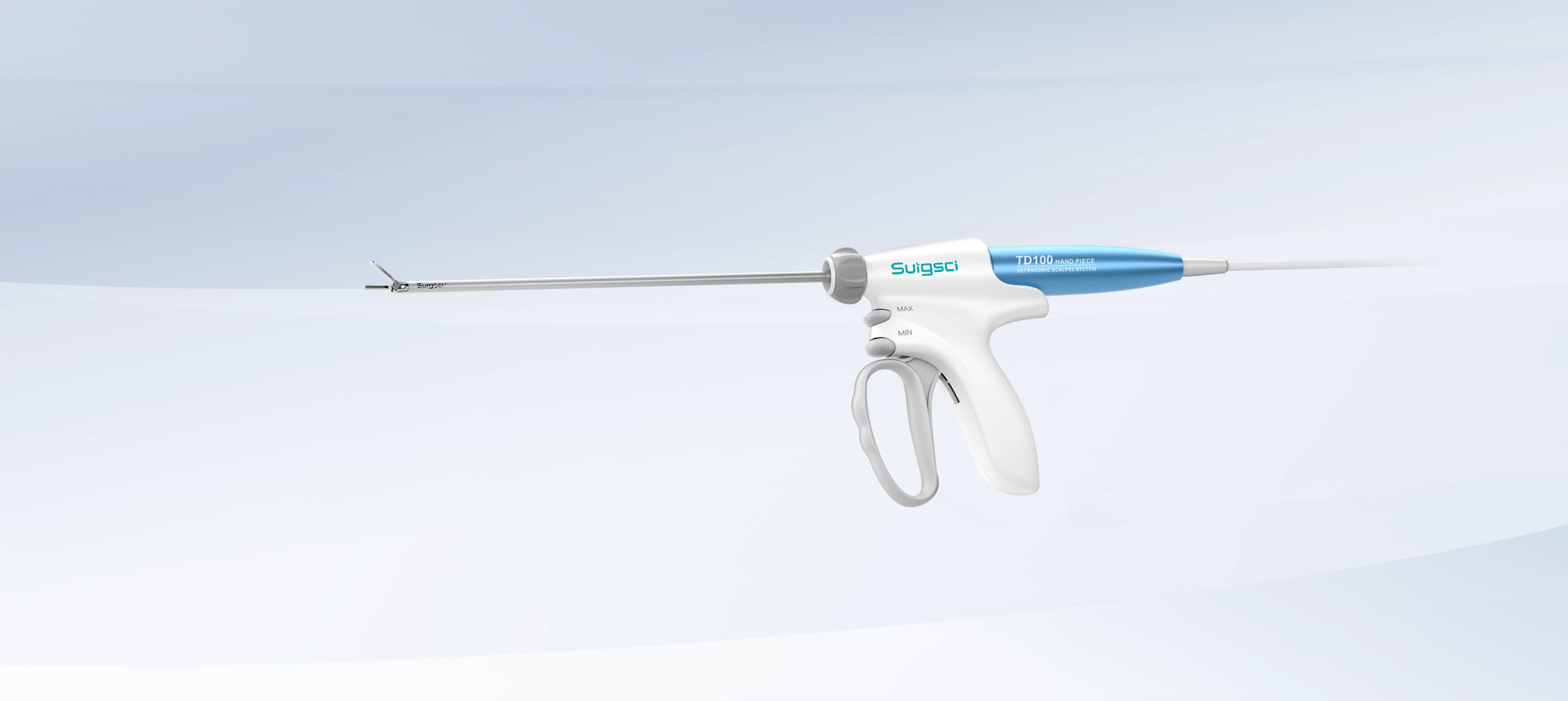
Recently, the Shenzhen Municipal Science and Technology Innovation Commission officially announced that the key technological research project titled “Research and Development of Key Technologies for a New High-Reliability, Anti-Adhesion Medical Ultrasonic Scalpel Tip”, led by Surgsci Medical Ltd., has successfully passed its final acceptance review.
The project focused on four major areas: a) Development of specialized titanium alloy materials for ultrasonic scalpel tips; b) Research on anti-tissue adhesion surface treatment technologies for scalpel tips; c) Acoustic structural design of new ultrasonic scalpel shafts; d) Achievement of reliable vessel sealing for diameters ≤7mm.
This milestone not only highlights the strong R&D capabilities of Surgsci Medical Ltd., but also marks a significant advancement in the core technologies of domestically produced ultrasonic scalpels.
Research Background and Significance
Ultrasonic scalpels are among the most widely used instruments in minimally invasive surgery, capable of simultaneously cutting tissue and achieving hemostasis. They play an irreplaceable role in clinical practice. However, domestic products have long been constrained by limitations in core materials and surface treatment technologies, making it difficult to compete with imported brands.
Against the backdrop of rapid global market growth, overcoming these technical bottlenecks is not only critical to the development of the industry but also essential for enhancing China’s independent innovation and international competitiveness in the high-end medical device sector.
Research Achievements and Breakthroughs
Through systematic research and rigorous validation, the project has achieved several key breakthroughs:
Materials: The team successfully developed ultra-long, ultra-fine grain titanium alloy manufacturing technology, delivering exceptional fatigue resistance that meets international standards. This provides a solid foundation for the production of domestic ultrasonic scalpel waveguide rods.
Processes: A stable and reliable surface treatment process was established, forming a biomimetic anti-adhesion structure on the scalpel tip. This effectively addresses the issue of tissue adhesion during surgery, which can reduce procedural efficiency.
Structure: The team re-engineered the compatibility between the scalpel tip and the waveguide rod, significantly improving cutting efficiency and coagulation performance. Experimental validation confirmed the system’s reliability and safety under clinical conditions.
Performance: By integrating advanced titanium alloy properties, anti-adhesion surface technology, proprietary waveguide design, and AI-based control algorithms, the system achieved effective vessel sealing for diameters up to 7mm.
Conclusion and Outlook
The successful acceptance of this project is a testament to the dedication and innovation of the Surgsci R&D team. It also fills critical technological gaps in the development of domestic ultrasonic scalpels. With comprehensive breakthroughs in materials, processing, and structural design, the product now offers enhanced reliability and clinical value.
Looking ahead, Surgsci Medical Ltd. remains committed to its mission of “improving health and quality of life.” Guided by clinical needs, the company will continue to empower physicians and benefit patients by transforming scientific achievements into real-world clinical solutions—driving China’s high-end medical devices onto the global stage.